How are White LEDs Made?

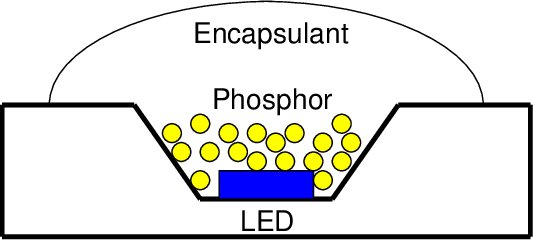
Blue to White Conversion by Phosphor in a Chip-on-Board (COB) LED
White LED lamps are made using an LED phosphor on top of UV or blue light emitting diode. The light emitting diode (LED) converts electricity to high energy light. The phosphor material or film converts the high energy light from the LED to lower energy (longer wavelength) light. Phosphors can absorb all or part of the light emitted by the LED. As a result, phosphors then produce color wavelengths longer from that of the absorbed light. Without phosphors, it is generally very difficult and expensive to produce broadband or white light from LEDs. Light emitting diodes without down-conversion phosphors emit a narrow band light and white light requires a broadband spectrum.
How is White Light Produced from LEDs?
The various breakthroughs achieved in the efficiency of UV/blue LEDs helped make phosphor-converted LEDs (pcLEDs) possible. Such pcLEDs are now a serious contender for conventional incandescent bulbs used in illumination and display backlighting applications. Most commercially available white LED lamps work by converting a portion of the blue LED emission to yellow. Some of the blue light from the LED is transmitted through the phosphor and mixed with the yellow phosphor emission, thereby resulting in a perceived white light.
The most common inorganic phosphor used in commercial white LEDs is the cerium-doped yttrium aluminum garnet Y3Al5O12:Ce (YAG:Ce) and its various derivatives. However, in recent years, several new types of inorganic-based fluorescent materials have also been used. Organic-based (fluorescent) materials and organic LEDs (OLEDs) that work by electroluminescence are also available. However, organic molecules are more susceptible to deterioration and accelerated aging. This is particularly true when exposed to intense UV or blue light and when subjected to the high temperatures present in some applications. Inorganic phosphors are manufactured at temperatures exceeding 1000 degrees centigrade. Therefore, phosphors tend to be very stable in comparison with organic-based systems.
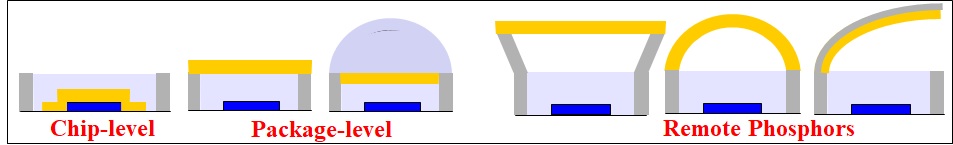
LED Phosphors (illustrated in yellow) and their various placement on blue (or UV) light-emitting diodes
There are many different shades of white light possible with phosphor-converted LEDs or pcLEDs. Those are generally categorized by the correlated color temperature (CCT), color rendition index (CRI), or color fidelity index (Rf). LED phosphor materials play a crucial role in the ability of a white LED to deliver white light colors having a specific CCT, CRI, or Rf. Phosphors can also determine how much deep red and near-infrared light a white LED is emitting. Standard white LEDs do not include any infrared (IR) light in their spectrum, while the sunlight spectrum is about 55% IR. IR light is invisible to the human eye but it is still used beneficially by trillions of living cells for energy production in the mitochondria. ATP, or adenosine triphosphate, is the primary energy molecule produced by the mitochondria within a cell when exposed to near-infrared light. The use of IR light in ATP energy production is true in human, animal, and even plant cells but to a lesser degree.
Since IR light is invisible to the eyes, its value is generally underestimated when it comes to lighting products. A light bulb or fixture is generally valued and marketed based on how much white light brightness (lumens) it produces. Being invisible, IR light doesn’t contain any lumens but it still delivers energy fundamental to life and biology. There is currently an on-going campaign to incorporate deep red and infrared light into white LED bulbs. You can watch a 1-minute video illustration below.
